GELATIN
● What is Gelatin?
- Comprised of 18 different kinds of amino acids, gelatin is a high molecular weight protein extracted with collagen hydrolysis which is a natural protein that consists of the skin, bone, tendon, cartilage, and other parts of animals.
- The raw material of gelatin includes bovine hide, bone, fish scales, and others, and it is classified into either type A gelatin complete with the acid treatment process or type B gelatin processed with an alkali treatment depending on the raw material treatment processes.
- If dissolved in warm water, gelatin turns into a sol state, and upon the cooling of the sol, it develops into a gel state with elasticity at a room temperature. Due to its reversible state transformation characteristics depending on the temperature, gelatin is widely used in food and pharmaceutical industries.

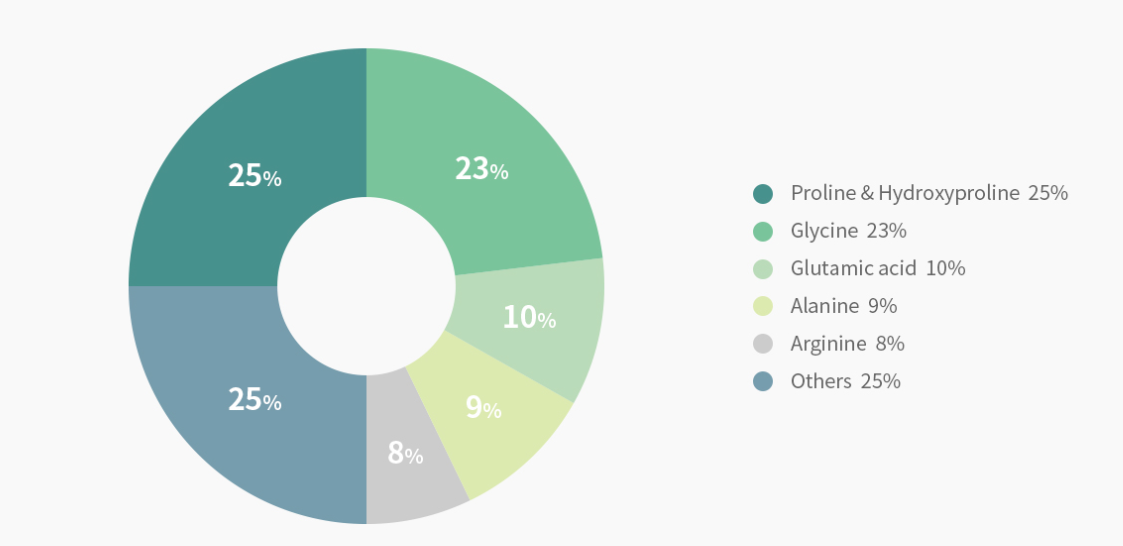
● Gelatin amino-acid composition
● Physical Properties of Gelatin
▶ Gel Strength
- The value of gel strength is measured when a 6.67% concentration of gelatin is left at 10°C for 16-17 hours. Along with the viscosity, gel strength is one of the most significant properties of gelatin, and is affected by the temperature, pH, gelatin concentration, etc.
▶ Viscosity
- The viscosity of gelatin is determined by the time taken by 6.67% concentration gelatin to pass the pipette at 60°C. Generally, the viscosity of the gelatin solution is around 20-60mps. Factors affecting the viscosity include pH, temperature, and concentration, and the viscosity is at its lowest at an isoelectric point. The viscosity of the gelatin solution is lowered at a high temperature, and the rate of decrease in the viscosity is significantly affected by the temperature of the gelatin solution.
▶ Viscosity Breakdown
- Thermal degradation (decrease in the gel strength and viscosity) of the gelatin solution occurs at a high temperature. The degree of thermal degradation is the function of the temperature, time, and pH, and the degradation especially occurs at an extreme acidic or alkaline condition and at 60°C or over.
▶ pH
- The pH range of the commercial gelatin is 5.0-6.0, and gelatin is stable against heat if the pH is roughly around neutral. Bacterial growth is inhibited at a low pH.
▶ Isoelectric Point
- The isoelectric point is the pH of a gelatin solution in which the ions are completely removed. There is no ionic movement at the isoelectric point, and the solution may get hazy as well. This phenomenon especially occurs at a low concentration and with gel state solutions.
● Application
▶ Edible Gelatin
- Gelatin enters into a sol state at a temperature of 35°C or over and a gel state if the temperature is lower than 35°C. Because of its melting point that is lower than the body temperature, gelatin melts easily inside the mouth, and due to its elasticity, it has been widely used to make various kinds of processed food such as jelly but also chocolate, gum, dessert, etc. Also, gelatin has a high moisturizing activity so it is often used to make gummy candy, marshmallow, ham, sausage, and others, and it also functions as a stabilizer in yogurt and ice cream. Thus, the application range of gelatin is extremely broad in the food industry.
▶ Pharmaceutical Gelatin
- Since gelatin forms a film at room temperature, it prevents the oxidation of contents and blocks moisture from the outside. Moreover, as gelatin is easily dissolved and absorbed inside the human body, it has been efficiently used in the pharmaceutical industry. In particular, gelatin is a common material for manufacturing hard capsules, soft capsules, tablets, etc.
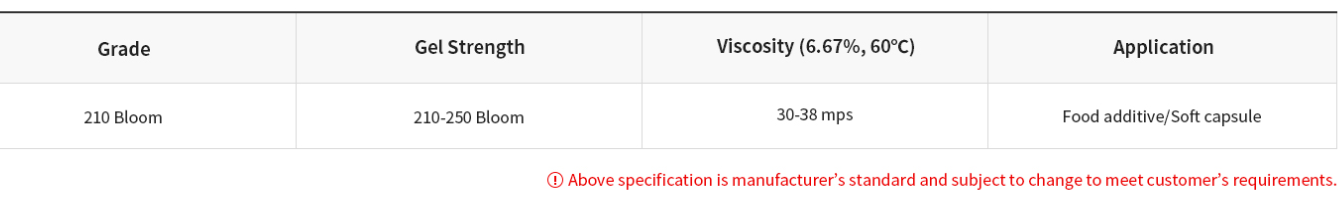
● Product List
▶ Bovine Hide Gelatin
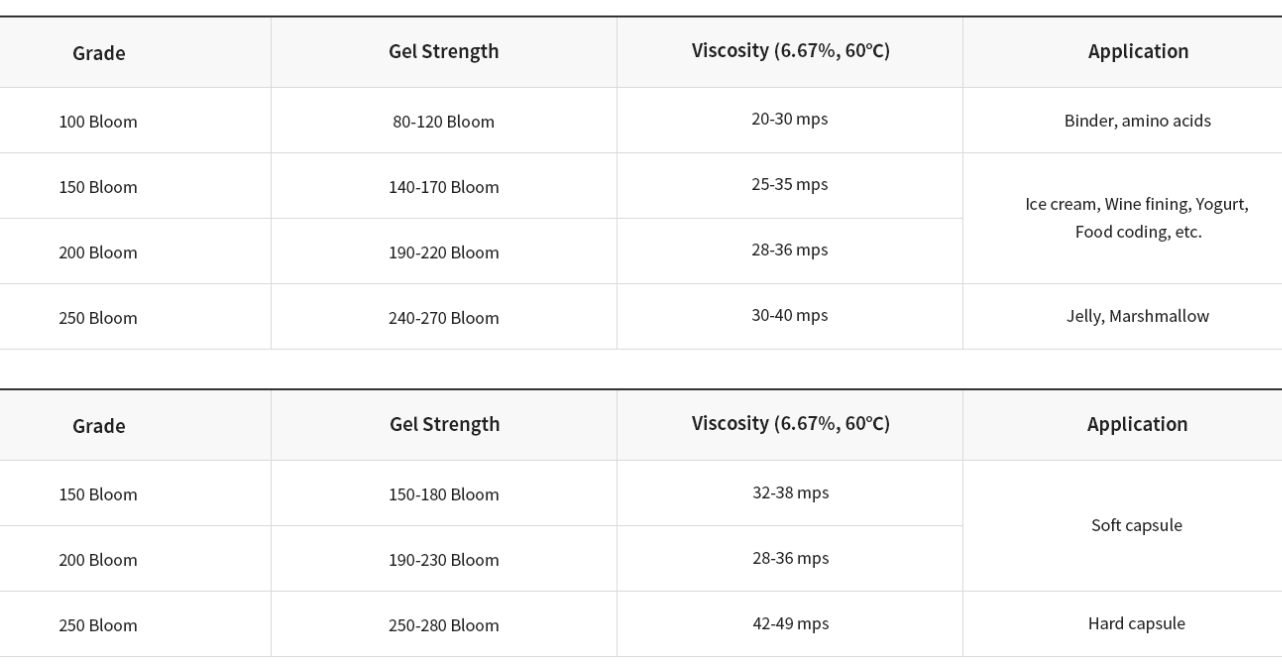
▶ Bovine Bone Gelatin
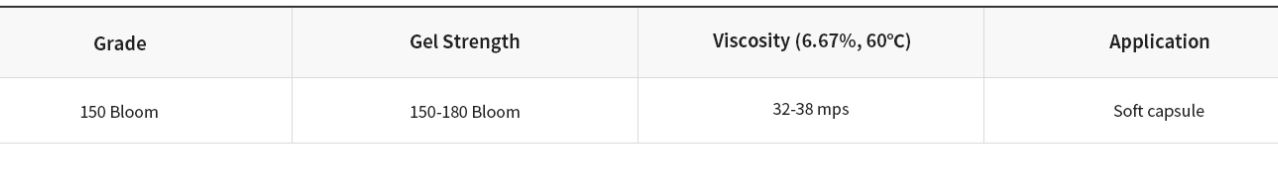
▶ Fish Gelatin