Saccharin
- Saccharin is a high intensity, artificial sweetener that has been used for over one hundred years as a sugar substitute. Saccharin tastes over 500 times sweeter than sugar which means that it can be used in small amounts to reduce sugar consumption.
- Saccharin has no calories and a Glycemic Index (GI) of zero. Saccharin is not absorbed or broken down by the body and has no effect on blood sugar levels. It is therefore considered as an important sugar substitute to help combat diabetes and obesity. Saccharin is also heat stable. Under conditions of increasing heat, saccharin remains stable at temperatures up to at least 250°C. Therefore, saccharin is commonly used in candies, cookies, some formulations of soft drinks as well as in mouth washes, toothpastes and as part of the tablet coating in medicines. We also produce the saccharin that is used to make table top sweeteners.
● Manufacturing Process
- R&F Process, the optimal method for producing high quality Saccharin
- Saccharin has been extensively studied and both the US FDA and EPA have conclusively declared it safe for consumption. Current global health standards only regulate saccharin for impurities based on the Remsen-Fahlberg synthesis route, first developed over 100 years ago. However, many other manufacturers use an alternative synthesis route that can give rise to other impurities and by-products. Saccharin made by the alternative route can therefore comply with the standards but still contain significant impurities. JMC manufactures saccharin in a completely vertically integrated process, via the Remsen-Fahlberg route, using mainly water-based processes and we manufacture all starting materials ourselves. We have on-site analysis facilities that test for all possible contaminants.
- In summary, JMC has over 65 years of production experience which enables us to deliver the world’s highest quality saccharin.
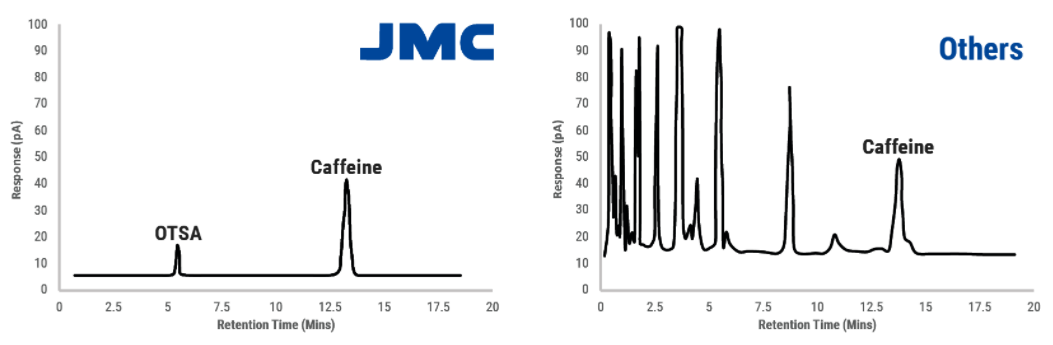
● Self-Produced raw material
- High quality Saccharin comes from the high purity raw material
- JMC’s R&F Process requires OTSA (Ortho-Toluenesulfonamide) as a raw material, while other processes use a different raw material, PA (Phthalic Anhydride) that is more prone to forming impurities during reactions. JMC is one of the few companies in the world to produce OTSA and our completely vertically integrated process enables us to control the purity at every step.
● Global Standard
- Saccharin complies with all Pharmacopoeias & Food Standards
- JMC Saccharin conforms to all Pharmacopoeias & Food standards (USP/NF, FCC, JECFA, EP, E954, JP, KP etc.). Currently all global standards are identifying and rating the quality of Saccharin based on JMC’s material. JMC saccharin is the global standard. JMC Saccharin is free from any allergen, irradiation, pesticides, GMO, BSE/TSE, gluten, latex and lactose even though these are not all regulated in the current Pharmacopoeias.
In their annual report, the USP Council of Experts have highlighted their current review of saccharin regulations. Catherine M. Sheehan, M.S., M.S. Senior Director, Science – Excipients, USP, is quoted (page 15):
Most people don’t think much about excipients, which are considered the ‘inactive’ ingredients in medicine. But these are actually critically important to how well a drug works in the body, as well as how it tastes if it’s oral medication. And they can cause great harm to patients if their quality is poor, so we are very proud of the work we do to help ensure the quality of excipients. We published draft standards seeking comments from international stakeholders revising the standards for three types of saccharin. Today, saccharin is used to help make medicines—which would otherwise taste pretty bad—palatable for patients. It is also used as an excipient for medications delivered intravenously or by aerosol, which means it’s critical to control impurities. This work is important because we know that saccharin may contain about 10 impurities that can develop during some manufacturing processes, including a few that can be harmful. This is part of our work with the Pharmacopeial Discussion Group, which includes USP, the European Pharmacopoeia, and the Japanese Pharmacopoeia, to harmonize excipient monographs and general test methods in order to align standards to bring more efficiency in quality testing.
● No Impurities
- No room for impurities in JMC Saccharin
- Since JMC uses high purity OTSA and the R&F Process – the optimal method for high quality Saccharin, there are no impurities in JMC Saccharin. By comparison, other Saccharin producers use different raw materials and processes that lead to a wide range of impurities.
● No Organic Solvents
- Refined with potable water
- JMC Saccharin is manufactured and processed using only potable water. This eliminates organic solvents as possible contaminants. JMC thoroughly manages the quality of the water used in our processes as well as in our waste streams.
| Product | Structural Formula | Downloads | Use |
|---|---|---|---|
| Sodium Saccharin 15% moisture CAS # 6155-57-3 CAS # 82385-42-0 CAS # 128-44-9 | 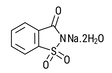 | Specification Sheet |
|
| Sodium Saccharin 6% moisture CAS # 6155-57-3 CAS # 82385-42-0 CAS # 128-44-9 | 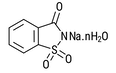 | Specification Sheet | |
| Insoluble Saccharin CAS # 81-07-2 | 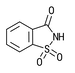 | Specification Sheet |