Aluminum sulfate
● Changes in Raw Materials
- Since Professor Wilber of Rutger University announced the results of his research on the cohesion characteristics of aluminum sulfate in 1885, aluminum sulfate has become the most widely used cohesion agent because of its cohesion characteristics and marketability.
Even nowadays, the standard plan for the treatment process of cohesion precipitation and its efficiency is fully based on the data from the cohesion precipitation of aluminum sulfate.
Aluminum sulfate used to be manufactured through a complicated process of filtering, discoloration and concentration after the reaction of sulfate with materials such as clay and the remains of alumina, alunite and bauxite.
Recently, high-quality aluminum hydroxide has become the major raw material in the development of the aluminum industry, High-quality aluminum hydroxide contains almost no impurities and produces pure aluminum sulfate when it directly reacts with sulfate.
● Manufacturing Methode
Since 1985, our company has been producing aluminum sulfate and selling it to meet a wide range of demands on different levels. We have adapted well to changes in demand in recent years and to the elevation of quality standards through technological innovation. Due to our consistent quality management effort, we have acquired a KS M1411 certification and are now producing a most reliable product.
By market demand, our company is now shifting from producing solid aluminum, sulfate to liquid aluminum sulfate. We are thus extending our manufacturing equipment for liquid aluminium sulfate, even as we are manufacturing products with different particle size.
Reaction equation) 2Al(OH)₃ + 3H₂SO₄ + nH₂O → Al₂(SO₄) ․ nH₂O
● Quality and Standard
KS M 1411 stipulate quality specification of aluminum sulfate as follows, General industry and tap water specification as follows.
● Industry
item | Industry 3rd grade |
|---|
KS M1411-1987 | Our Spec. |
|---|
Appearance | - | - |
pH(2W/V%) | 3.0 or more | 3.0 or more |
Water insoluble solids(%) | - | - |
Al₂O₃(%) | 8.0 or more | 7.0~8.2 |
(Fe)(%) | 0.02 or below | 0.02 or below |
● Tap Water(Liquid)
Item | Tap water |
|---|
KS M-1987) | Our Spec. |
|---|
Appearance | - | - |
pH(2W/V%) | 3.0 or more | 3.0 or more |
Water insoluble solids(%) | - | - |
Al₂O₃(%) | 8.0 or more | 8.0 or more |
(Fe)(%) | 0.02or below | 0.02or below |
Ammonia nitrogen(%) | 0.01or below | 0.01or below |
(As)(%) | 10.0or below | 10.0or below |
(Mn)(%) | 25.0or below | 25.0or below |
(Cd)(%) | 2.0or below | 2.0or below |
(Pb)(%) | 10.0or below | 10.0or below |
(Hg)(%) | 0.2or below | 0.2or below |
(Cr)(%) | 10.0or below | 10.0or below |
● Physicochemical Characteristics
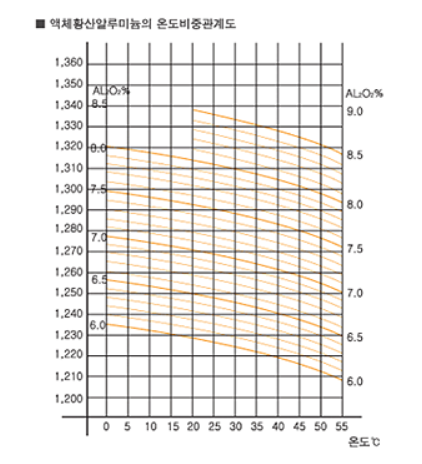
1. Relationship with Specific Gravity, Temperature and Concentration
The genuine specific gravity of solid aluminum sulfate is about 1.62, but its temporary specific gravity is 0.6~0.7. the graph below shows the relationship among the specific gravity, the temperature and the concentration(%) of liquid aluminum sulfate. The chart will determine the sulfate's concentration(%).
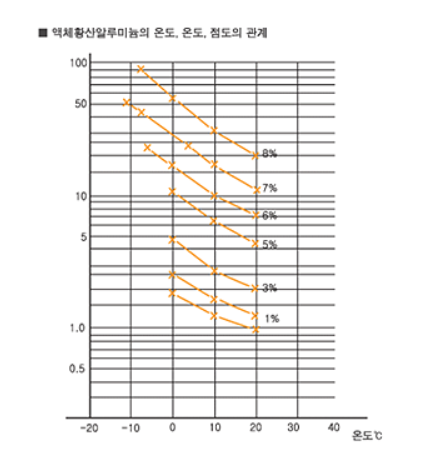
2. Mucosity
Refer to the chart for the relationship between the concentration and the mucosity of aluminium sulfate.
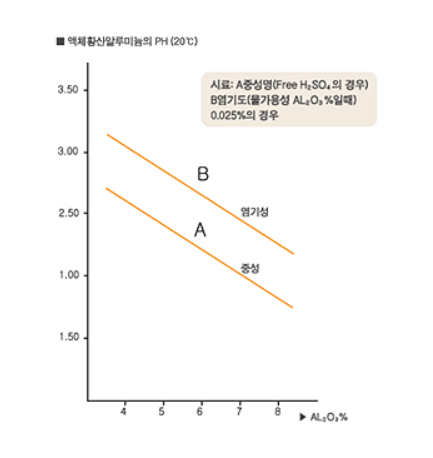
3. pH
Refer to the chart for the relationship between the valid ingredient of the neutral salt/acid solution and the pH [at 20 ℃] of the liquid aluminum sulfate sample : A- when it is neutral salt, B- when its basicity is 0.025%.
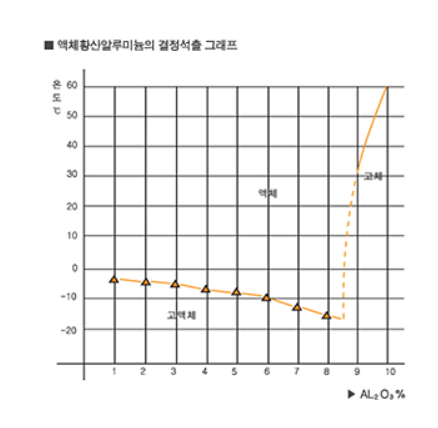
4. Crystallization Temperature
The crystallization temperature of liquid aluminium sulfate is lowest[about -14 C] when its concentration is less than 8%. Its crystallization temperature rises dramatically, however, when its concentration is greater than 8.2%, and it finally closes pipes/devices in concentration. Therefore, the concentration of the product in the market is either 7.0% or 8.0%.
5. Chemical Characteristics
The composition of aluminium sulfate can be expressed, and solid alu\-minium sulfate is expressed, as Al₂(SO₄).nH₂O due to the crystallization of water molecules. In Korea, products with 14-16 molecules are generally manufactured.
These are colorless crystals that are soluble in water but in soluble in alcohol. The hydro soluble liquid extracts the precipitation of hydroxy aluminium, which is acidic. If the solution is boiled for a long period of time, basic aluminium sulfate forms.
Al₂(SO₄)3·nH₂O → Al₂(SO₄)₃ 3· nH₂O → Al₂(O)3· 3SO₃
● JAR Test Method
After Putting the 500ml testing solution in the 500ml beaker, add the reagent continuously using the mess pipette while Stirring at 100~120 rpm. Rapid stirring for 1~2minutes is continued for dispersion.
Continue slow stirring at 50~60rpm for 10minutes for FLOC growth, and stop stirring for 5~6 minutes.
Measure the size and precipitation state of FLOC and the turbidity, pH, COD and BOD using the upper 100ml, to determine the best condition.
1. Adjustment of the Turbidity, pH and Alkalinity of the Original solution before its addition. ② Stirring – The condition mentioned above is standard.
2. Stirring – The condition mentioned above is standard.
3. If an organic macromolecular agglutinating agent is used together with the reagent, the reagent is added first and stirred for 10~60 seconds. Then the organic macromolecular agglutinating agent is added and the mixture is stirred slowly but continuously.
4. Adjustment of the Aluminum Sulfate Solution for the Selt-test.
● The Table below shows the amount of agglutinating agent that should be added to the diluting solutions
ppm | 500cc(ml) | 100cc(ml) | 1m³(ml) |
|---|
1% sol | 10% sol | 1% sol | 10% sol | 1% sol | 10% sol |
|---|
1 | 0.05 | 0.005 | 0.1 | 0.1 | 10 | 1 |
2 | 0.10 | 0.01 | 0.2 | 0.2 | 20 | 2 |
3 | 0.15 | 0.015 | 0.3 | 0.3 | 30 | 3 |
4 | 0.20 | 0.02 | 0.4 | 0.4 | 40 | 4 |
5 | 0.25 | 0.025 | 0.5 | 0.5 | 50 | 5 |
6 | 0.30 | 0.03 | 0.6 | 0.6 | 60 | 6 |
7 | 0.35 | 0.035 | 0.7 | 0.7 | 70 | 7 |
8 | 0.40 | 0.04 | 0.8 | 0.8 | 80 | 8 |
9 | 0.45 | 0.045 | 0.9 | 0.9 | 90 | 9 |
10 | 0.50 | 0.05 | 1.0 | 0.1 | 100 | 10 |
20 | 1.0 | 0.1 | 2.0 | 0.2 | 200 | 20 |
30 | 1.5 | 0.15 | 3.0 | 0.3 | 300 | 30 |
40 | 2.0 | 0.2 | 4.0 | 0.4 | 400 | 40 |
50 | 2.5 | 0.25 | 5.0 | 0.5 | 500 | 50 |
60 | 3.0 | 0.3 | 6.0 | 0.6 | 600 | 60 |
70 | 3.5 | 0.35 | 7.0 | 0.7 | 700 | 70 |
80 | 4.0 | 0.4 | 8.0 | 0.8 | 800 | 80 |
90 | 4.5 | 0.45 | 9.0 | 0.9 | 900 | 90 |
100 | 5.0 | 0.5 | 10.0 | 1.0 | 1000 | 100 |
● Uses
1. Water purifier : national water supply, industrial water supply, industrial waste water, metropolitan sewage
2. Sizing agent for paper mills
3. Die production
4. Figment production
5. Precipitant for ceramic materials
6. Pharmaceutical production
7. As a raw material for various aluminum compounds
● Instructions for Storage
The inside of the container should be resistant to corrosion using a rubber lining, FRP, an epoxy resin or polyethylene. In case of leakage, neutralize thoroughly with slaked lime, baking soda and calcium carbonate.
1. Handling Precautions
Wash the substance off immediately if it stains clothing. Otherwise, the clothing will be damaged. Wash the substance off completely if it comes in contact with tour skin. If you leave it as it is for a long period of time, it will roughen your skin. Wash it off immediately if it comes in contact with your eye. If the situation is serious, consult your eye specialist. Wearing a safety glass is a very good idea.
2. Storage of Aluminum sulfate in winter
Aluminum sulfate can freeze in winter due to the evaporation phenomenon on the inside wall of the tank. As the liquid temperature goes down, the nuclei of Al₂(SO₄)₃․28H₂O goes up and begins to close the pipe laying. Therefore, keep in mind the following facts goes up and begins to close the pipe laying. Therefore, keep in mind the following facts.
3. Insulation
Keep the pipe laying, the valve, the pump and the small tank warm. (Be especially careful with parts exposed to outside elements. If possible, keep them warm using a steamer/heater.)
4. Dilution
Check the specific gravity of the aluminum sulfate at around November, and dilute it under 1.327/20 if it is above 1.327/20.
5. Insertion of Low-concentration Products
In cold areas, freezing can occur if the pipe laying is the pipe laying is thin or distant, due to the slow flow rate. In this case, the insertion of a low-concentration product could be helpful.
→ Give us a call if the substance still freezes after the above instructions are performed.